

Eye Morphogenesis

Retinal Development

Eye Evolution
A major focus our research program is to elucidate the molecular and cellular control of optic cup morphogenesis, and we have focused significant effort on the choroid (optic) fissure region of the eye. During eye development, the optic primordia undergo a complex series of morphogenetic movements that ultimately result in a bilayered optic cup containing the prospective retina and retinal pigment epithelium (RPE). The neuroectodermal layers of each optic primordium must fuse along its proximo-distal axis such that the retina and RPE will be confined to the optic cup during the early phases of ocular morphogenesis. Fusion occurs at a distinct region of the optic cup called the choroid fissure (CF). Defects in CF closure result in colobomas, congenital malformations of the eye. Despite a significant amount of genetic research to identify coloboma loci, causative mutations have been identified in less than 20% of coloboma patients. Moreover, we lack a comprehensive understanding of the cell biological and morphogenetic mechanisms underlying CF closure in the human eye, in any of the animal model systems utilized for modeling human eye development and disease, or in stem cell models of optic cup formation. Previous studies in our laboratory have identified roles for the Hh pathway during optic vesicle patterning that enable CF closure (Lee et al., 2008; Lee et al., 2012), a requirement for laminin-containing extracellular matrix during CF closure (Lee et al., 2007), and we identified Bcl6a as a direct Vax2 target in the ventral optic cup, determining that it acts with several of its transcriptional co-factors (BCOR, Hdac1, Rnf2) in preventing apoptosis in the ventral optic cup during CF closure (Lee et al., 2013). Our work has lead to a model in which choroid fissure closure occurs in three stages (Fig. 1). During Stage 1, retinoblast proliferation generates sufficient cells such that, as optic cup morphogenesis proceeds, the lateral edges of the CF are brought into close apposition. During Stage 2, the basement membrane (BM) lining the CF is degraded, and this is facilitated, in part, by the hyaloid vasculature (Hartsock et al., 2014; James et al., 2016), which then enables adhesion between cells lining the opposing sides of the fissure. During Stage 3, cells on opposing sides of the fissure form adhesions that spread and thereby close the CF. While much has been learned about Stage 1, we know virtually nothing about how BM breakdown and tissue fusion occur during CF closure, and these are the areas on which we are now focused.


Recently, we also embarked on a study examining the function of Mitf-family transcription factors and optic cup morphogenesis, as mutations in in MITF, result in colobomas in human patients (George et al., 2016). Utilizing a novel mitfa;tfec mutant line, we first demonstrate that loss of these Mitf-family transcription factors phenocopies colobomas observed in MITF patients. Our data indicate that both RPE and cNCC development is perturbed in mitfa;tfec mutants. Through a series of embryological manipulations and rescue experiments, we determined that Mitf-family function is required specifically within the cNCCs to facilitate CF closure. Further, our data suggest that Mitf transcription factors act within cNCCs to promote localization in and around the eye and cell survival. Taken together, these data identify potential cellular underpinnings of colobomas in human COMMAD patients with mutations in MITF and provide a platform through which cNCC-specific functions during CF closure can be further elucidated (Sinagoga et al., 2020).

Previous work in our laboratory focused on the regulation of proliferation and differentiation in retinal progenitor cells (retinoblasts) and within the stem cell containing ciliary marginal zone (CMZ) of the retina. We demonstrated that de novo purine synthesis regulates retinoblast proliferation, wherein an ATP-dependent pathway modulates cell cycle kinetics in proliferative retinoblasts (Ng et al., 2009). We identified a mutation in cluap1, a component of the IFT-B complex, and showed that cluap1 mutants have defects in ciliogenesis that result in photoreceptor degeneration (Lee et al., 2014). We performed a series of studies on the HLH protein, Id2a, and the gene regulatory network in which it operates. Id2a modulates retinoblast cell cycle kinetics and thereby influences eye growth, as well as neuron and glia formation in the retina (Uribe and Gross, 2010). Id2a acts upstream of the Notch pathway in regulating cell cycle progression and neuronal differentiation, and via RNA-Seq, we have identified numerous additional downstream Id2a effectors (Uribe et al., 2012). In collaboration with Peter Hitchcock (University of Michigan), we also showed that Id2a functions downstream of the secreted factor Midkine-a during retinoblast proliferation and differentiation (Luo et al., 2012). Finally, through a series of studies on the zebrafish patched1 mutant (formerly patched2) we identified roles for patched1 in regulating the size of the retinal progenitor pool in the CMZ (Bibliowicz and Gross, 2009), and in preventing ectopic proliferation and retinal dysplasia in the post-embryonic retina (Bibliowicz and Gross, 2011).

A major focus in our lab currently is on DNA methylation (5mC) and hydroxymethylation (5hmC) during eye development and, specifically, during the transition from retinal progenitor cell (RPC) to differentiated retinal neuron and in maintenance of retinal stem cells (RSCs). Our long-term goal is to generate genome-wide, single-nucleotide resolution 5mC and 5hmC profiles for RPCs of different competency states and for specific types of differentiated retinal neurons, and to correlate these profiles with quantitative gene expression data and then determine whether 5mC and 5hmC marks are required for normal gene expression and cell fate transitions in these cells. We hypothesize that epigenetic regulation of gene expression within RPCs and RSCs facilitates normal retinal development and growth by influencing the ability of these cells to remain as progenitors or to differentiate as retinal neurons. While a growing body of work demonstrates that epigenetic factors play key roles in retinal development, and possibly retinal disease, we do not yet understand how changes in DNA methylation or hydroxymethylation in RPCs and RSCs facilitate continued proliferation or differentiation, nor do we know whether these changes in methylation and hydroxymethylation are required for normal development or continued retinal growth and neurogenesis. While our genome data will be instrumental in predicting how epigenetic changes affect gene expression during RPC maturation and differentiation, and RSC maintenance, we need a mechanism to functionally test these predictions. Thus, we have systematically characterized and generated mutations in the genes that effect 5mC to 5hmC conversion (the tet2- and tet3 dioxygenases; Seritrakul and Gross, 2017), the maintenance methyltransferase, dnmt1 (Tittle et al., 2011; Angileri and Gross, 2020), and the genes encoding zebrafish de novo DNA methyltransferses, which methylate naked cytosines (the Dnmt3-family members; Seritrakul and Gross, 2014). We anticipate that the results of these studies will be of broad interest to the retinal development field, and translationally, for the continued development of stem cell-based therapies aimed at repairing or regenerating cells lost to disease or injury.
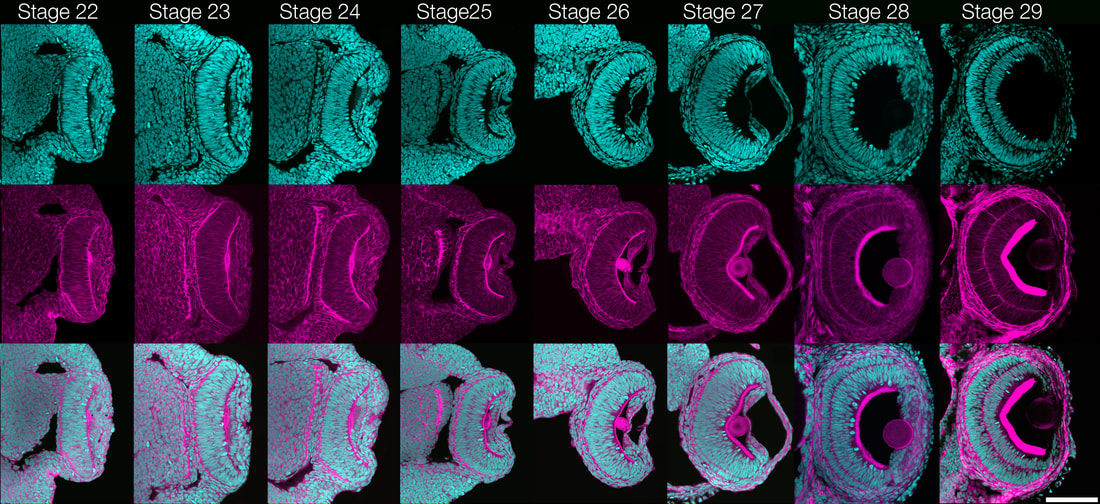
To better understand the eye of the Urbilaterian ancestor and how complex eye morphologies can evolve, it is essential to establish a Lophotrochozoan model for the development of a cephalic, complex image-forming eye. We have chosen to build off of the rich history of embryology and neurobiology using Loligo pealeii, and further develop it as a model Lophotrochozoan for studies of eye evolution (Koenig et al., 2016). Although Loligo pealeii has been the subject of developmental biology research for over 50 years, molecular analyses are in their infancy in the system. Research in the Cephalopod eye has mainly focused on mature tissues or on Pax-6 as a master regulator of eye specification. Gene products involved in patterning the developing eye and regulating morphogenesis remain almost entirely unknown. Our focus has been to develop Loligo pealeii as a system where developmental, molecular and evolutionary studies can be performed to elucidate the molecular basis of eye formation in the Lophotrochozoa, and by extension, across the Bilateria. To achieve this, we have generated a substantial amount of “infrastructure” for genomic and molecular studies, and are now using these data for more directed experiments on eye formation. For example, we generated a staging series that covers all stages of embryonic eye formation, a fate map of the early eye field, and we performed a series of detailed studies on cell proliferation, cell death, and retinal organization (Koenig et al., 2016). There are no published Cephalopod genomes, making molecular studies slow and tedious. To address this problem, we sequenced and assembled the Loligo pealeii transcriptome, generating over 100 gigabases of transcript sequence, which assembled into 72,591 isogroups with an N50 value of 1178 bp. This project has enabled us to make a substantial leap forward in beginning to identify the conserved regulatory network underlying eye development across the Bilateria. Indeed, we have taken both a candidate gene approach, having identified and characterized conserved regulators of eye development in Drosophila and vertebrates (e.g. the Pax6 cascade, Hh pathway, Notch pathway, and numerous other transcriptional regulators), and we have utilized RNA-Seq on dissected eyes and optic lobes from five stages of development to identify potentially novel regulators of eye development in Loligo pealeii. Finally, we are performing functional analyses of some of these conserved and novel factors, and our data support a role for the Notch pathway in regulating proliferation and differentiation in the Cephalopod retina (Koenig et al., 2016).
Ongoing work focuses on developing projects in other organisms where we can leverage their unique biology to uncover fundamental aspects of eye development and potentially develop these into new therapeutic approaches to treat human eye diseases (Koenig and Gross, 2020). Loligo pealeii, and further develop it as a model Lophotrochozoan for studies of eye evolution (Koenig et al., 2016). Although Loligo pealeii has been the subject of developmental biology research for over 50 years, molecular analyses are in their infancy in the system. Research in the Cephalopod eye has mainly focused on mature tissues or on Pax-6 as a master regulator of eye specification. Gene products involved in patterning the developing eye and regulating morphogenesis remain almost entirely unknown. Our focus has been to develop Loligo pealeii as a system where developmental, molecular and evolutionary studies can be performed to elucidate the molecular basis of eye formation in the Lophotrochozoa, and by extension, across the Bilateria. To achieve this, we have generated a substantial amount of “infrastructure” for genomic and molecular studies, and are now using these data for more directed experiments on eye formation. For example, we generated a staging series that covers all stages of embryonic eye formation, a fate map of the early eye field, and we performed a series of detailed studies on cell proliferation, cell death, and retinal organization (Koenig et al., 2016). There are no published Cephalopod genomes, making molecular studies slow and tedious. To address this problem, we sequenced and assembled the Loligo pealeii transcriptome, generating over 100 gigabases of transcript sequence, which assembled into 72,591 isogroups with an N50 value of 1178 bp. This project has enabled us to make a substantial leap forward in beginning to identify the conserved regulatory network underlying eye development across the Bilateria. Indeed, we have taken both a candidate gene approach, having identified and characterized conserved regulators of eye development in Drosophila and vertebrates (e.g. the Pax6 cascade, Hh pathway, Notch pathway, and numerous other transcriptional regulators), and we have utilized RNA-Seq on dissected eyes and optic lobes from five stages of development to identify potentially novel regulators of eye development in Loligo pealeii. Finally, we are performing functional analyses of some of these conserved and novel factors, and our data support a role for the Notch pathway in regulating proliferation and differentiation in the Cephalopod retina (Koenig et al., 2016).
Ongoing work focuses on developing projects in other organisms where we can leverage their unique biology to uncover fundamental aspects of eye development and potentially develop these into new therapeutic approaches to treat human eye diseases (Koenig and Gross, 2020).